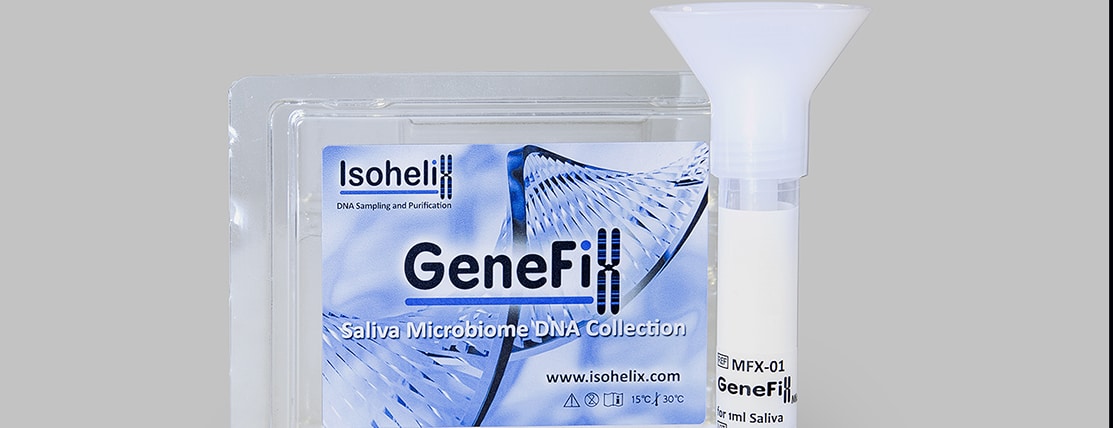
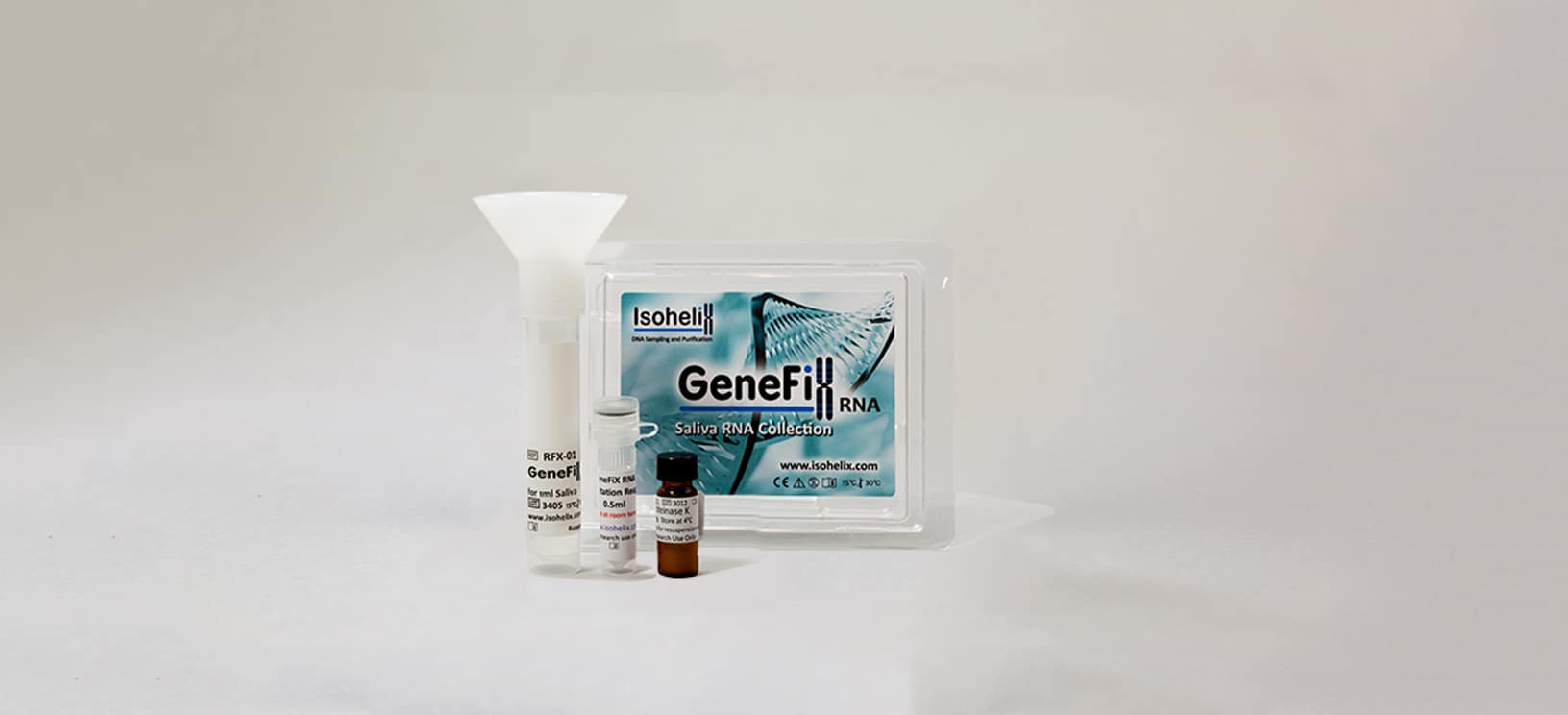
We are very excited to welcome Nina to the team at Isohelix. Nina brings a wealth of experience in life science sampling and customer focus, and will be working face to face with our customers in the UK and Europe, helping them to find the best solution to their DNA sampling workflows through our innovative sample collection, stabilisation and purification products.
Welcome Aboard Nina, Sales Manager – Europe