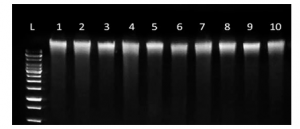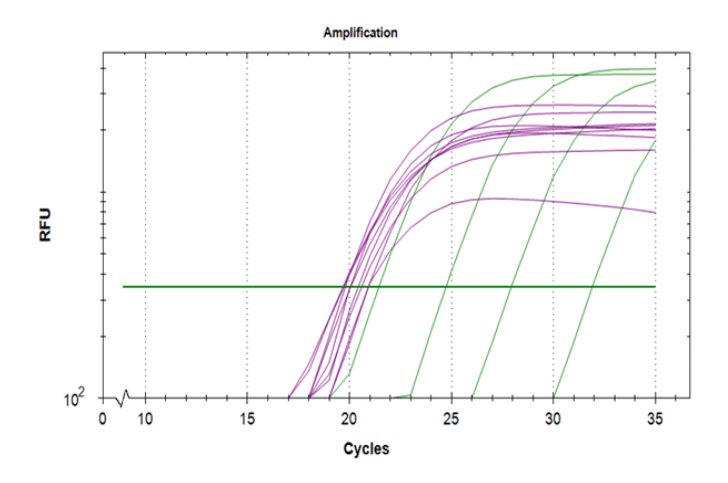
Obtaining high-quality RNA is the first step for sensitive downstream applications such as RT-PCR, digital PCR, and RNA-seq. However, this can be tricky, as RNA is fragile and susceptible to both physical degradation and digestion by ubiquitous ribonuclease (RNAse) enzymes.
In this blog, we give you ten top tips to ensure success when working with RNA to help you get the best results from your samples.
1. Use an area of the lab dedicated to working with RNA
RNases are ubiquitous in the environment and maintain their activity even after autoclaving, so make sure to decontaminate your reagents and equipment before using them. Set aside a separate area for working with RNA away from other work, and use separate pipettes, tips, and other consumables. Ideally, use a UV decontamination cabinet or laminar flow hood to prevent contamination from airborne micro-organisms. Your skin secretes RNases, so wear clean gloves and a clean lab coat and face mask while handling samples.
2. Decontaminate work surfaces and equipment
Common disinfectants such as 70% Isopropanol may be insufficient for inactivating RNases. Wipe down all work surfaces, and clean pipettes with a 10% household bleach solution (or a commercially available RNAse decontamination solution), followed by wiping down with RNAse-free/DEPC-treated H2O to inactivate potential RNases. Use filtered pipette tips for liquid handling to prevent aerosols and the cross-contamination of samples. Where possible, use sterile, disposable plasticware . If you must use non-disposable plasticware, treat it with 0.1 M NaOH/1 mM EDTA and RNase-free water. Always use molecular biology grade consumables and reagents certified as RNAse/Nuclease free, as lower quality reagents may be contaminated with RNases. ISOHELIX PRODUCTS ARE PRODUCED IN A CLEAN ENVIRONMENT AND ARE CERTIFIED RNAse-FREE
3. Use an RNA stabilization reagent
RNA collection and analysis from saliva and buccal swab samples is especially challenging, given the high quantities of enzymes in the oral cavity. To overcome this, Isohelix has developed unique, non-toxic RNA stabilization buffers and included them in RNA collection kits, immediately preserving RNA from the moment of collection :
- BuccalFix RNA Stabilization and Lysis Kits– fully stabilize RNA at room temperature for up to four weeks.
- GeneFix RFX– stabilizes RNA in saliva samples for up to two months at room temperature.
Using an RNA stabilization reagent preserves the integrity of the RNA in your saliva and buccal swab samples during room-temperature storage and shipping.
4. Handle samples carefully
Many RNA extraction kits include guanidium isothiocyanate (GITC) during the lysis stage to denature proteins. However, GITC is a toxic reagent that can potentially react with bleach (sodium hypochlorite) to generate toxic gases.
Xtreme-RNA is free from toxic reagents such as phenol, chloroform, β-mercaptoethanol, & guanidine salts, so it is much safer to handle in the lab.
5. Choose an RNA Extraction Kit optimized for your sample type
Choose the most appropriate kit for your samples and downstream applications. For example, the Isohelix Xtreme-RNA kit is a spin column based RNA purification kit for the swift, simple preparation of total human, viral, or microbial RNA, optimized for extraction from saliva and swab samples. Extracted samples are of high purity (expected A260/280: >1.9), so they are ideal for use in downstream applications such as rt-qPCR, RNAseq and microRNA-seq. The kit is scalable and can accommodate various sample input volumes.
6. Avoid toxic reagents
Many RNA extraction kits include guanidium isothiocyanate (GITC) during the lysis stage to denature proteins. However, GITC is a toxic reagent that can potentially react with bleach (sodium hypochlorite) to generate toxic gases.
Xtreme-RNA is free from toxic reagents such as phenol, chloroform, β-mercaptoethanol, & guanidine salts, so it is much safer to handle in the lab.
7. Be aware of potential gDNA contamination
The similarity in physical and chemical properties of DNA and RNA makes gDNA contamination of isolated RNA a common problem, and non-specific amplification due to gDNA contamination of samples can lead to overestimates of transcript levels using RT-PCR gene expression analysis. Many RT-PCR assays can be designed to be gDNA insensitive using exon-spanning primers, but this is not always possible. If required, DNase digestion can be performed following the final elution of RNA using most commercially available DNAse kits.
8. Accurately quantify your RNA
Before proceeding with downstream applications, assess the quality and quantity of extracted RNA. Use spectrophotometry (e.g., NanoDrop) for concentration determination and electrophoresis or capillary electrophoresis (e.g., Agilent Bioanalyzer) to assess RNA integrity.
9. Work swiftly
RNA in inadequately maintained oral samples can be degraded by intracellular nucleases. Extract RNA as quickly as possible after taking samples, and once begun, work as fast as you can to complete sample purification. Purified samples should always be kept chilled or on ice.
10. Always freeze extracted RNA
Even trace amounts of RNase can degrade RNA in non-frozen samples. Store extracted RNA short-term at -20°C (weeks to months), but in the longer term, store RNA at -80°C (years) as the low temperature will inhibit enzyme activity, preventing sample degradation.
More Info
To find out more about Isohelix’ High Quality Products for Collecting, Stabilizing and Extracting RNA, click here RNA Products | Isohelix